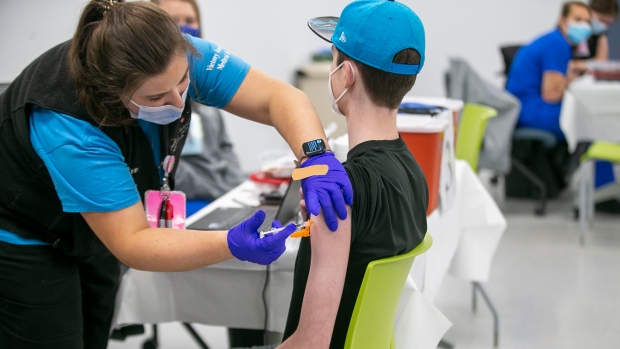
OTTAWA -- Canada is watching the U.S. Food and Drug Administration closely now that it has approved Pfizer-BioNTech's COVID-19 vaccine for children aged five to 11.
The FDA approved the vaccine for emergency use on Friday afternoon, citing data that shows the shot is 90 per cent effective in preventing COVID-19 in children and there have been no serious side-effects.
The FDA advisory committee recommended in favour of approving the vaccine after hours of testimony from experts Tuesday.
Health Canada officials attended that meeting and are watching the process in the U.S. carefully as Canada makes its own decision about the pediatric vaccine.
“Those deliberations and the discussions will be inputs into our review as well,” said chief Health Canada medical adviser Dr. Supriya Sharma at a briefing Friday.
Health Canada received Pfizer-BioNTech's submission for approval and associated data slightly later than the FDA, and Canadian officials are still reviewing it.
“Our scientists are in the process of looking at not only the results from the clinical trials and the studies that were done, but also the details on the new formulation,” Sharma said.
The pediatric COVID-19 vaccine differs slightly from Pfizer-BioNTech's adult formulation. For that reason, the company will need to deliver new vials of the vaccine before Canadian kids can get their shot.
Canada is expecting 2.9 million child-size doses of the new formulation if it is approved, enough for every child to get their first dose.
But Sharma said it could still be a few weeks until Health Canada makes a final decision.
“We've received some additional information just last week that we're looking through,” Sharma said.
“I think it's going it's going very well. I can't see a decision before mid to the end of November.”
The FDA and U.S. Centers for Disease Control and Prevention will be monitoring the rollout of the children's' vaccines for any adverse effects, and Canadian public health officials will be keeping an eye on that data as well.
The CDC will meet next week to discuss clinical recommendations in the administration of the children's vaccine in the U.S.
Meanwhile, Health Canada is expecting more submissions for pediatric vaccines in coming weeks.
Moderna will be submitting an application for a pediatric vaccine “soon,” according to Patricia Gauthier, the general manager of Moderna Canada.
Moderna's interim data for its vaccine for kids six to 11 shows a strong safety profile as well as a strong immune response, Gauthier said at a public event hosted by The Canadian Club on Tuesday.
“We now need to have like future analysis, but this is very positive which now leads us to be able to have conversations with Health Canada and start thinking about that submission,” she said.
This report by The Canadian Press was first published Oct. 29, 2021.
US clears child-size doses of Pfizer COVID-19 vaccine - CTV News
Read More

Tidak ada komentar:
Posting Komentar